Untitled Document
 |
|
Recruiting patients for drug trials in India is big business
|
India's outsourced call centres are well known, but not its outsourced
patients.
By 2010, some estimate there will be two million patients in India on clinical
trials.
An entire industry has sprung up, specialising in recruiting patients and managing
experiments.
And a BBC investigation into the conduct of these trials has found that some
patients are unaware they are being experimented on at all.
Most of the world's largest pharmaceutical companies have a presence in India,
but there is concern about how the country achieves its exceptional recruitment
rates and questions about fully-informed consent.
Medical language
Six years ago, an experimental drug from the US called M4N was injected
into cancer patients in India without being properly tested on animals first.
Later it was discovered that several patients had not known they were part
of a clinical trial.
 |
|
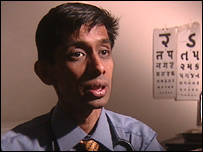
"Most of the patients sign on the dotted line without understanding the nature and the consequences of what is being administered to them"Dr Shashank Joshi
|
One of the doctors who later blew the whistle, Dr V Narayan Bhattathiri, told
the BBC: "I can only say that what they did is something unbelievable or
incomprehensible.
"I couldn't find any example of such a thing being done, maybe in the
last 50 years or so. Maybe something similar could have happened in say concentration
camps."
Giving informed consent to be part of an experiment is the golden rule of all
clinical trials which goes all the way back to the Nuremberg Code.
But one doctor at the prestigious Lilavati hospital in Mumbai, Dr Shashank
Joshi, says the idea of all patients giving informed consent in India is "a
myth according to me... because I do not think it's truly informed in the language
the patient understands.
"Most of the patients sign on the dotted line without understanding the
nature and the consequences of what is being administered to them."
Lack of understanding
Reporter Paul Kenyon tracked down a drug trial being conducted for a major
drug company in a psychiatric unit at a hospital in Gujurat.
It was to test an anti-psychotic drug developed by the world's second largest
drug pharmaceutical company Johnson and Johnson.
 |
|
"I didn't know that experiments were being carried out on me" Parshottam Parmar
|
There is already controversy over what is happening, with some doctors levelling
the accusation that patients are being taken off their existing medication as
part of the trial, with the potential they could suffer unnecessarily .
Dr Vikram Patel from the British Journal of Psychiatry says: "The most
obvious problem is that they won't get better or they will continue to suffer
this extremely severe psychiatric illness, much longer than they need to."
But the ethical concerns go deeper when Kenyon finds a patient who took part
in the trial.
"I was just told that the drugs were American. They used to give me the
tablets and I used to eat them," says Parshottam Parmar.
"We just sign because I believe the doctor takes the signature to help
us. That's why I sign it."
He says he had no idea that he was part of a clinical trial.
"I didn't know that experiments were being carried out on me. I was told
that the old drugs were discontinued and were no longer available in the pharmacies.
"I don't know a lot about all these things. I am poor and I live in a
small hut and I don't understand many things. The doctors are intelligent. They
write the drugs for me so I have to take them accordingly."
Johnson and Johnson's spokesman Dr Vivek Kusumaker told us: "We have looked
at this particular trial and we've got consent from the patient or from a relative
in every case.
"If there is any instance brought to our attention that something was
not OK we will take that seriously. We have said that we shut down sites if
we don't think we are carrying out research to the highest code of ethics in
which we believe."

